
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Kit Pengangkutan Virus Babi entuk Clearance FDA 510 (K)
2025-10-30
Kit Pengangkutan Virus Babi entuk Clearance FDA 510 (K)
Jinan, China - 2025 Oktober- Bioteknologi Babi, Produsen Cina sing misuwur saka reagen diagnostik medis, kanthi bangga ngumumake manawaKit Pengangkutan Virus (ora ora aktif)wis nampaFDA 510 (K) Clearance (K250205), kanthi resmi validasi selaras karo standar pangaturan A..
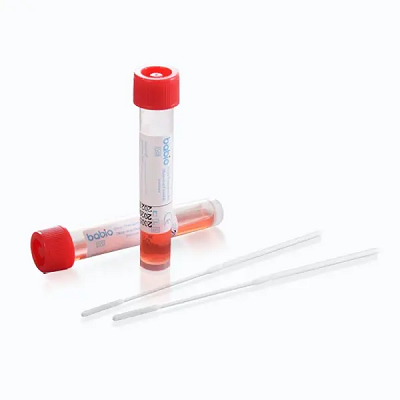
Tonggota tonggak iki nguatake pimpinan global Babi ing solusi transportasi spesimen klinis. Kit kasebut direkayasa kanggo koleksi virus sing aman lan stabil, aplikasi hilir sing ndhukung kayataRt-pcr, Budaya Virus, landiagnosa molekulWaca rangkeng-. Fitur:
-
✅ Stabilitas suhu kamar
-
✅ Solusi Hank sing Apik Kanthi Antibiotik
-
✅ Tabung conical Dnase-Gratis
-
✅ Safet Lemak Ngrampungake Kanthi Desain Breakpoint
Kasedhiya pirang-pirang volume isi (1mL-6ML) lan ukuran paket (20-500 tabung), kit kasebut cocog kanggo rumah sakit, laboratorium, lan institusi kesehatan umum ing saindenging jagad.
Karo aKapabilitas produksi saben dina 100.000 unit, Babi njamin sumber sing konsisten lan rega kompetitif. Dipercaya ingAmerika Serikat, Jerman, lan luwih, Babi terus ngirim inovasi lan linuwih ing sistem transportasi virus.learn liyane ing https://www.biocorp.com.
#Virustransportkit # fda510k #bio #clinicalicaliagnostics #vtm # rt-pcr #globalchealthcare #specimencollection #specimencollection


